Registered clinical trials investigating ketamine & esketamine for treatment-resistant depression
Journal of Psychedelic Studies | Published 16 January 2023
I’m excited to share my latest paper! The systematic review evaluating ketamine and esketamine (Spravato) clinical trials was recently accepted for publication in the Journal of Psychedelic Studies. Read more about it below:
Background & Aims: Ketamine and esketamine have garnered interest in both psychiatric research and clinical practice for treatment-resistant depression (TRD). In this review, we examined registered trials investigating the therapeutic use of ketamine or esketamine for TRD, with the aim of characterizing emerging trends and knowledge gaps.
Methods: The ClinicalTrials.gov electronic registry and results database was queried from inception to February 5, 2022, adhering to elements of the PRISMA guideline, we evaluated trial eligibility in the qualitative synthesis. Data regarding study design, drug regimens, and measures were subsequently abstracted and descriptively analyzed.
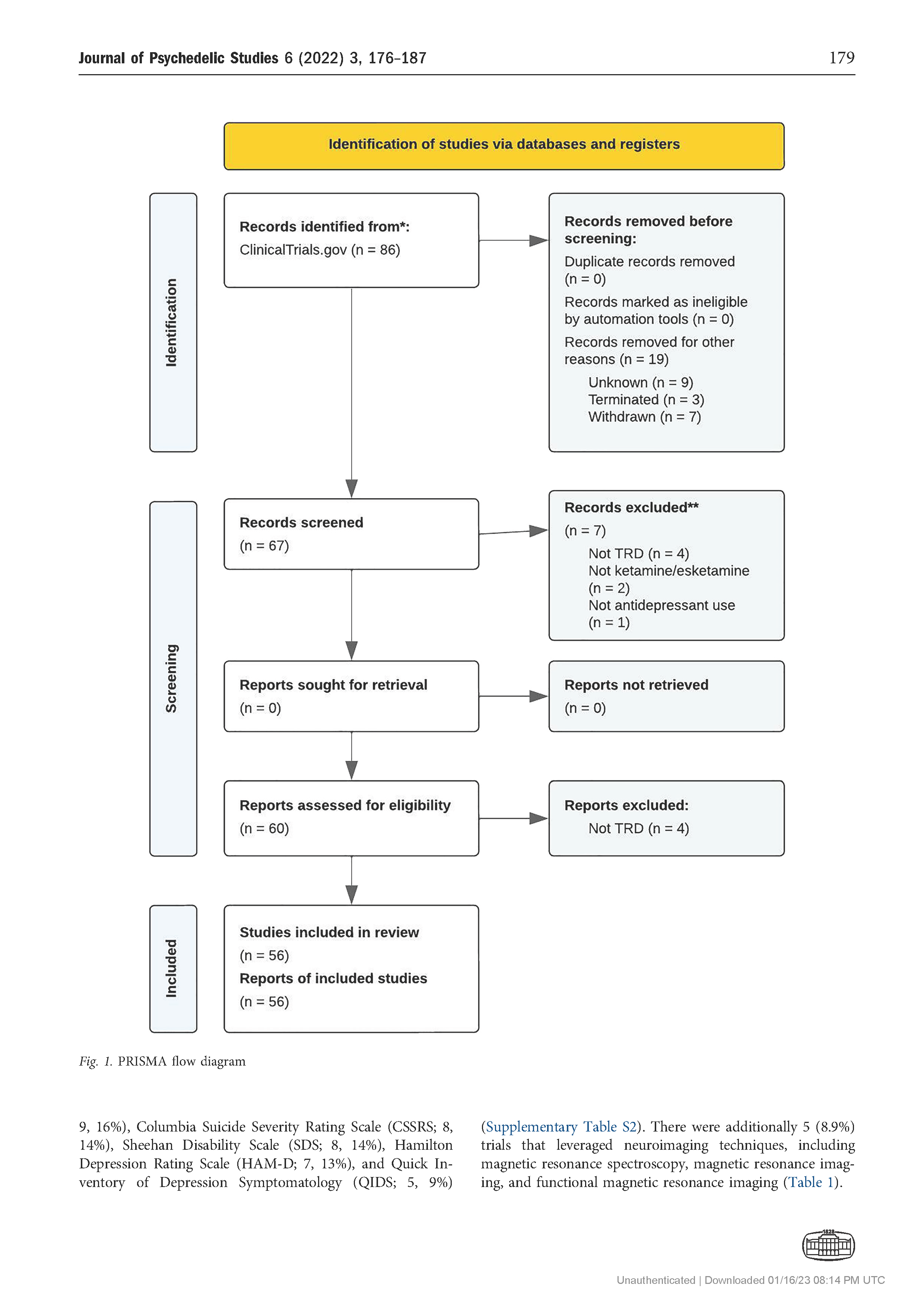
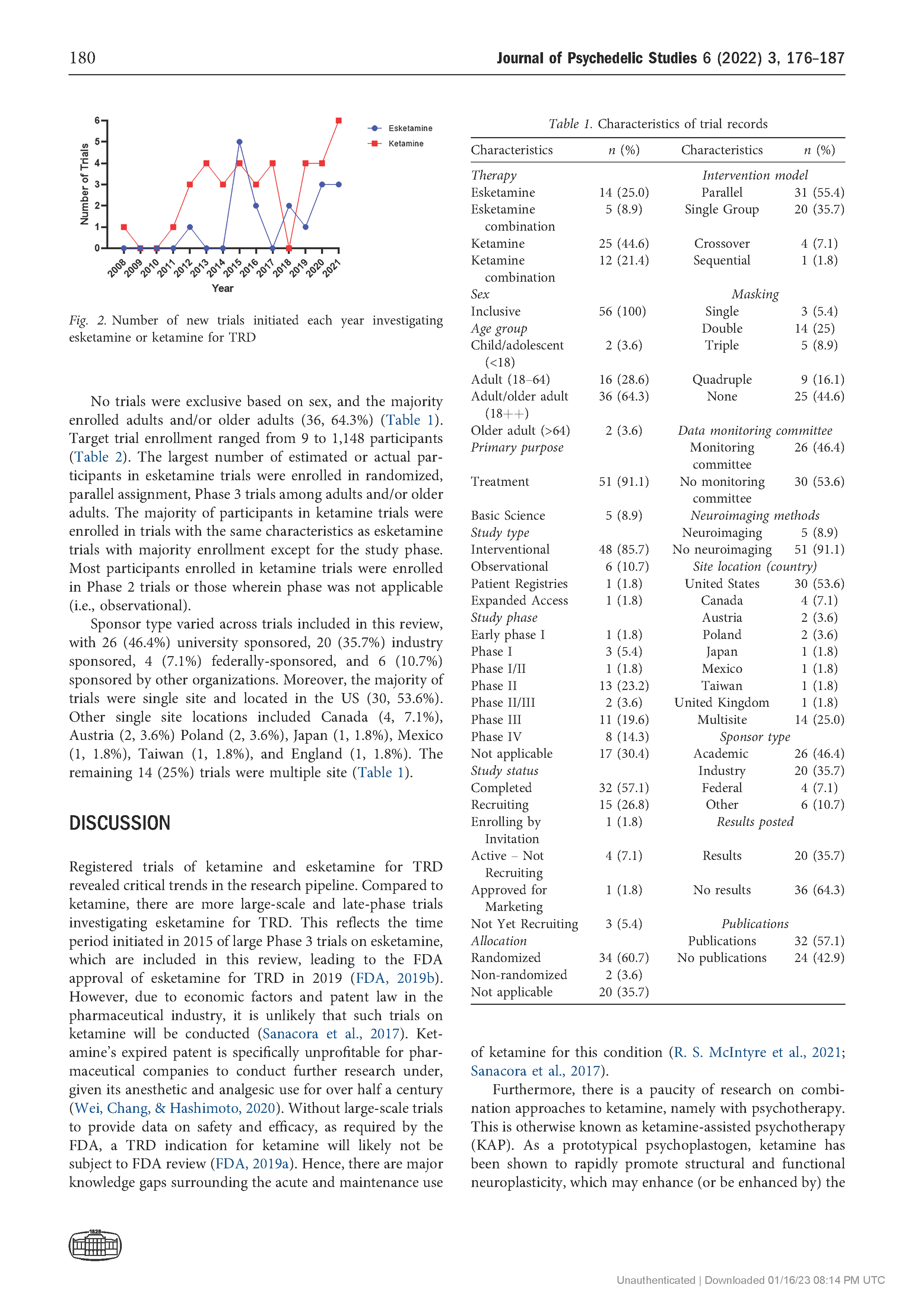
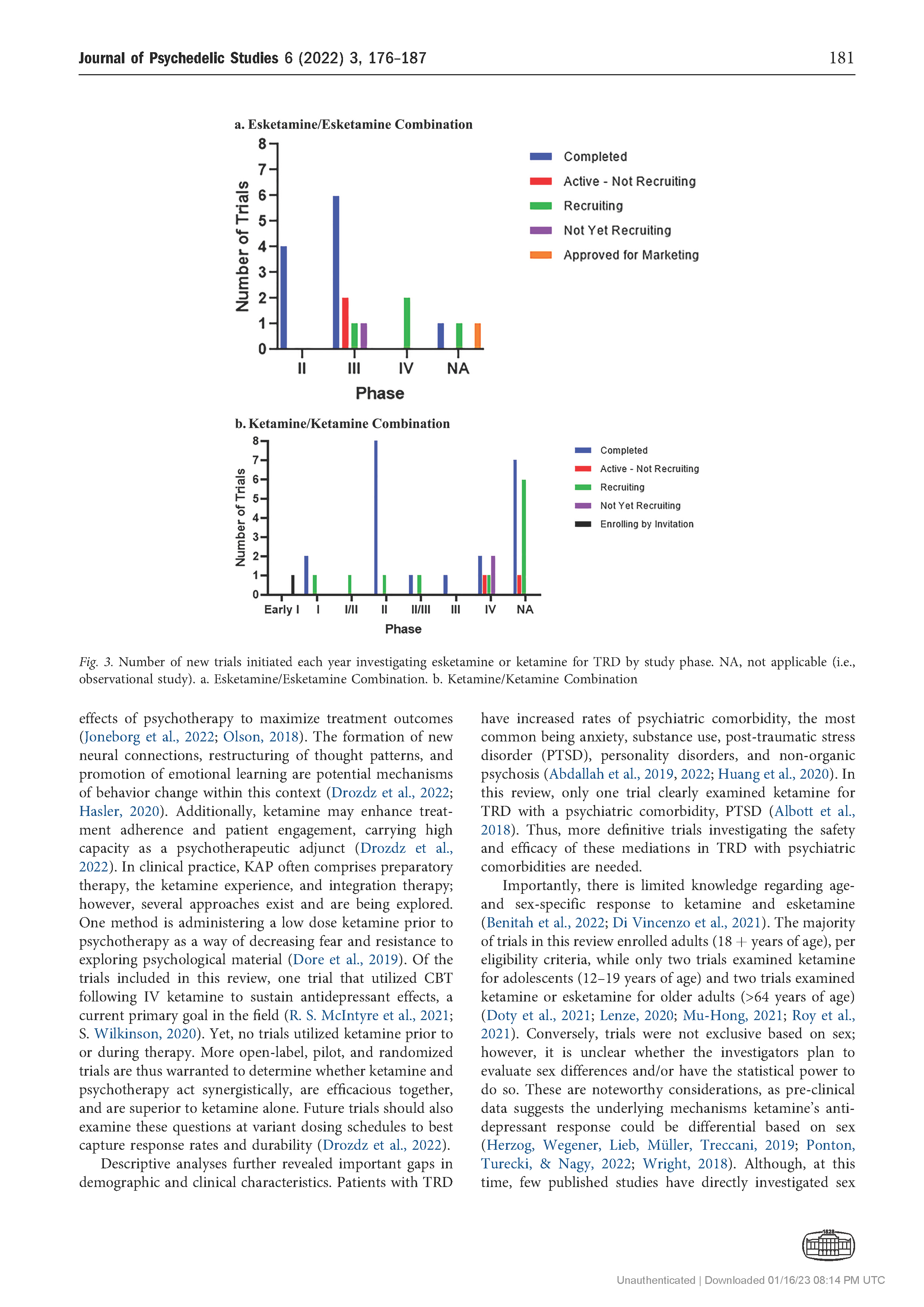
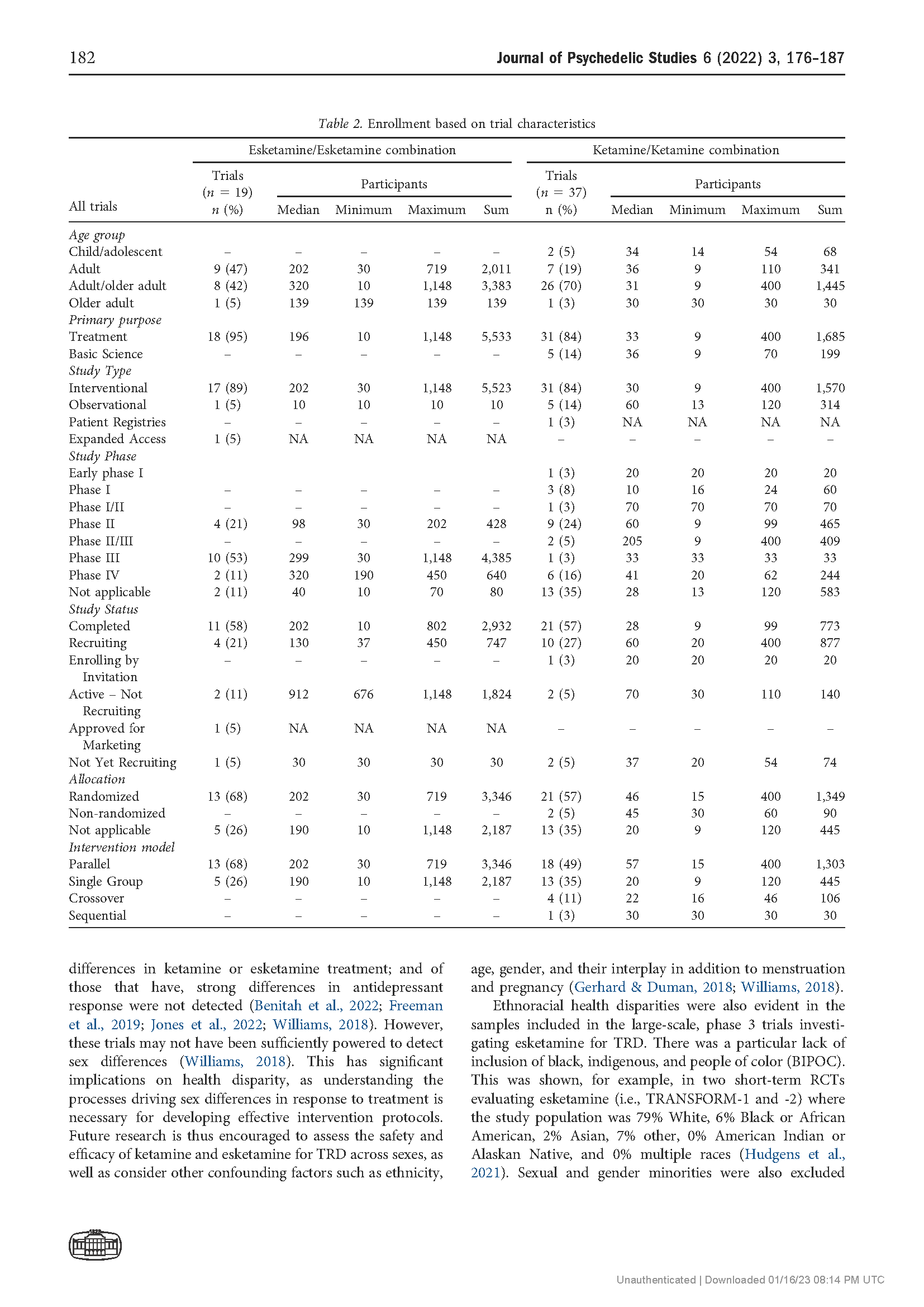
Results: The search returned 86 records, of which 56 trials were included in the final review. The number of trials investigating ketamine and esketamine for TRD increased since 2008, with higher peaks observed in 2015 (n 5 9) and 2021 (n 5 9). Most trials were Phase 2 (13, 23.2%) or Phase 3 (11, 19.6%), gathering preliminary data on efficacy and/or further data on safety and efficacy with variant dosing and pharmacological approaches. By and large, trials examined ketamine and esketamine as individual versus combination treatments (45% and 25%, respectively). The Montgomery-Asberg Depression Rating Scale (MADRS) was most commonly used to assess clinical outcomes (75%).
Conclusions: There are increasingly large-scale and late-phase trials of esketamine over ketamine for TRD, coupled with efforts to centralize evidence on these medications. Yet several trials do not assess patient characteristics that may affect treatment response, such as age, sex, and race. By understanding these design limitations, scientists and clinicians can avoid research waste and funding bodies can judiciously direct support towards high priority research.








Citation
Brendle, M., Ragnhildstveit, A., Slayton, M., Smart, L., Cunningham, S., Zimmerman, M. H., Seli, P., Gaffrey, M. S., Averill, L. A., & Robison, R. (2023). Registered clinical trials investigating ketamine and esketamine for treatment-resistant depression: A systematic review, Journal of Psychedelic Studies, 6(3), 176-187. doi: https://doi.org/10.1556/2054.2022.00234